Why Can't an Ionic Bond Form Between Potassium and Magnesium
When the solid dissolves the ions dissociate and can diffuse freely in solution. Were not gonna be able to form an Ionic bond because we have two positively charged ions positive and positive youre not going to attract in reality is they actually want to repel.

Water Pollution Facts About Chlorine Water Pollution Facts Water Pollution Chlorine
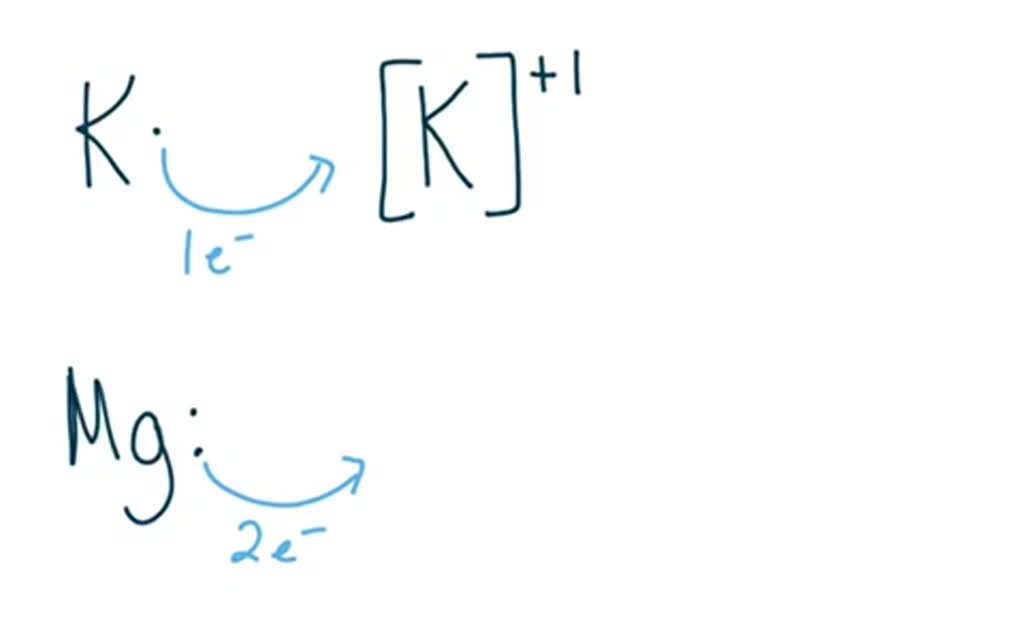
Potassium will form a 1 ion and magnesium will form a 2 ion.

. In this example the electrons are shown as dots and crosses. Sodium has only one valence electron while chlorine has seven. Why cant an ionic bond form between potassium and magnesium.
High electrical between cautions and anions in the crystal lattice therefore theres a high temperature is required to break down the lattice and change to a solid crystal into a liquid Why cant an ironic bond between potassium and magnesium. Therefore it will not. Lets explain why potassium does not bond with me on to For me compounds.
Why cant an ionic bond form between potassium and magnesium. The ions are atoms that have gained one or more electrons known as anions which are negatively charged and atoms that have lost one or more electrons known as. Oxygen is in group 6 of the periodic table.
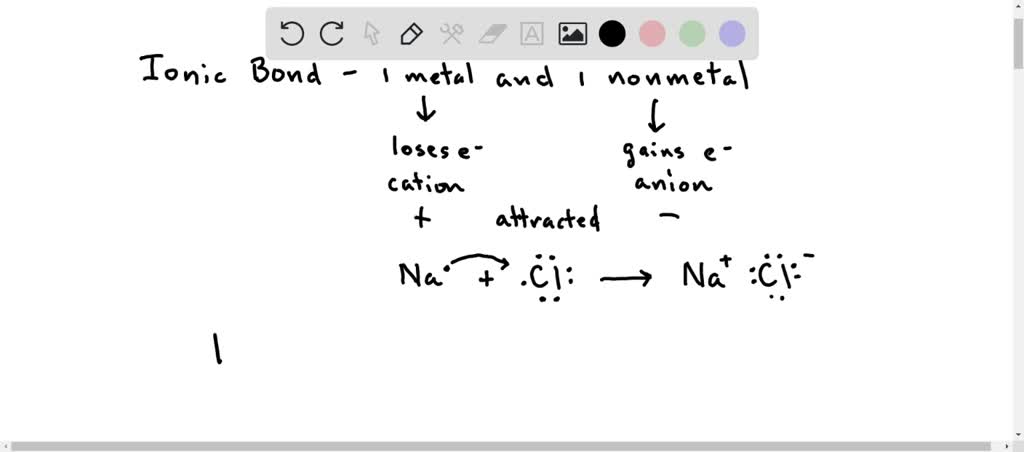
Ionic bonds are formed between two or more atoms by the transfer of one or more electrons between atoms. There must be one is metal and another one is non metalk mg both are metals. Ionic or polar covalent.
An ionic bond is a type of chemical bond where one or more electrons are transferred from one atom to another. It is conventional to show the cation without dots around the symbol to emphasize that the original energy level that contained the valence electron is now empty. Magnesium and potassium are the 2 major intracellular cations.
A metallic bond is formed when a metal and another metal bond. Potassium is a Group one medal that must donate one electron to achieve a stable configuration. Cuz they are both cations positive.
These arent oppositely charged therefore cant form an ionic bond. An oxygen atom will gain 2 electrons to form a stable 2-ion. Also there is nothing available to accept the electrons lost.
Answer 1 of 3. In the case of NaCl the difference in electronegativity between Na 093 and Cl 316 is 21. You will often see electrons drawn like this in books.
Potassium and magnesium are both metals so it forms a metallic bond. So an ionic bond forms between potassium and chlorine and Potassium Chloride gets formed. Electron transfer produces negative ions called anions and positive ions called cations.
Both elements are metals so react by losing electrons. A magnesium atom will lose 2 electrons to form a stable 2 ion. A nonmetal and metal form ionic bond.
A major function of potassium is. All potassium compounds are formed using ionic bonds. Thus the bond between Magnesium and oxygen is ionic.
Ted I on since now we have a positively charged potassium cat ion in a positively charged magnesium cattle on. This appears to result from an inability of the cell to maintain the normally high intracellular concentration of potassium perhaps as a result of an increase in membrane permeability to potassium andor inhibition of Na-K-ATPase. Cuz they have a strong bond which means it requires a lot of heat Why cant an ionic bond form between potassium and magnesium.
Repletion of cell potassium requires correction of the magnesium deficit. So in order for us to make an ion gone were gonna. Schellas form neon is unable to accept electrons.
The anion is now shown with a complete octet of electrons. What is the type of chemical bonding formed between potassium and iodine. Neon is a Group 18 noble gas that has a full older och tests of eight electrons and is completely stable.
The intracellular concentrations of these 2 ions appear to be closely correlated but the existence of a relationship between the plasma concentrations of these ions has been controversial. These ions can form when a metal reacts with a non-metal. An ionic bond is formed when a metal and nonmetal bond.
The ionic bond is the attraction of the Na ion for the Cl ion. These arent oppositely charged therefore cant form an ionic bond. These arent oppositely charged therefore cant form an ionic bond.
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions and is the primary interaction occurring in ionic compounds. Thats why ionic bond formation is. The ionic bond between magnesium and oxygen is stronger.
Because the electronegativity of chlorine is higher than the. Answer 1 of 8. For a compound such as magnesium chloride it is not.
Potassium will form a 1 ion and magnesium will form a 2 ion. To form a ionic bond there is conditions. Some anions may be large and the electron clouds may be diffuse or fluffy my term and a high positive charge may distort them.
Potassium will form a 1 ion and magnesium will form a 2 ion. Potassium atom has just one valence electron and Chlorine has 7 electrons Cl atom needs just one more electron to complete its octet which it receives from K atom. Forming ionic bonds Positively charged ions are called cations and negatively charged ions are called anions.
Right from the general names of the groups it can be understood that both Na and Mg. Ionic substances exist as crystalline solids. As a result the cells lose potassium which is excreted in the urine.
Na and Mg are metals belonging to the Group 1 alkali metals and Group 2 alkaline earth metals respectively of the Long Periodic Table. Magnesium is a metal found in grp 2 and oxygen is a nonmetal foumd in grp 6. Why cant an ionic bond form between potassium magnesium both are metals that form cations an ionic bond forms only between ions of opposite charges what is the difference between the chlorite ion the chlorate ion.
Both elements are metals so react by losing electrons.

Atomic Electron Shells Animated Presentation Youtube Nursing School Notes Teaching Science Homeschool Science

Ionic Or Electrovalent Compounds Properties Characteristics With Videos
Solved Why Can T An Ionic Bond Form Between Potassium And Magnesium
Solved Why Can T An Ionic Bond Form Between Potassium And Magnesium
No comments for "Why Can't an Ionic Bond Form Between Potassium and Magnesium"
Post a Comment